|
Dynamical origin of anomalous dynamics of supercooled water
|
We have investigated molecular origin of anomalous prperties of water
(dynamical origin of anomalous temperature-dependece of isobaric heat capacity,
structural and dynamical transition in deeply supercooled water and origin of
persisistent dynamics and low glass transition temperature of water).
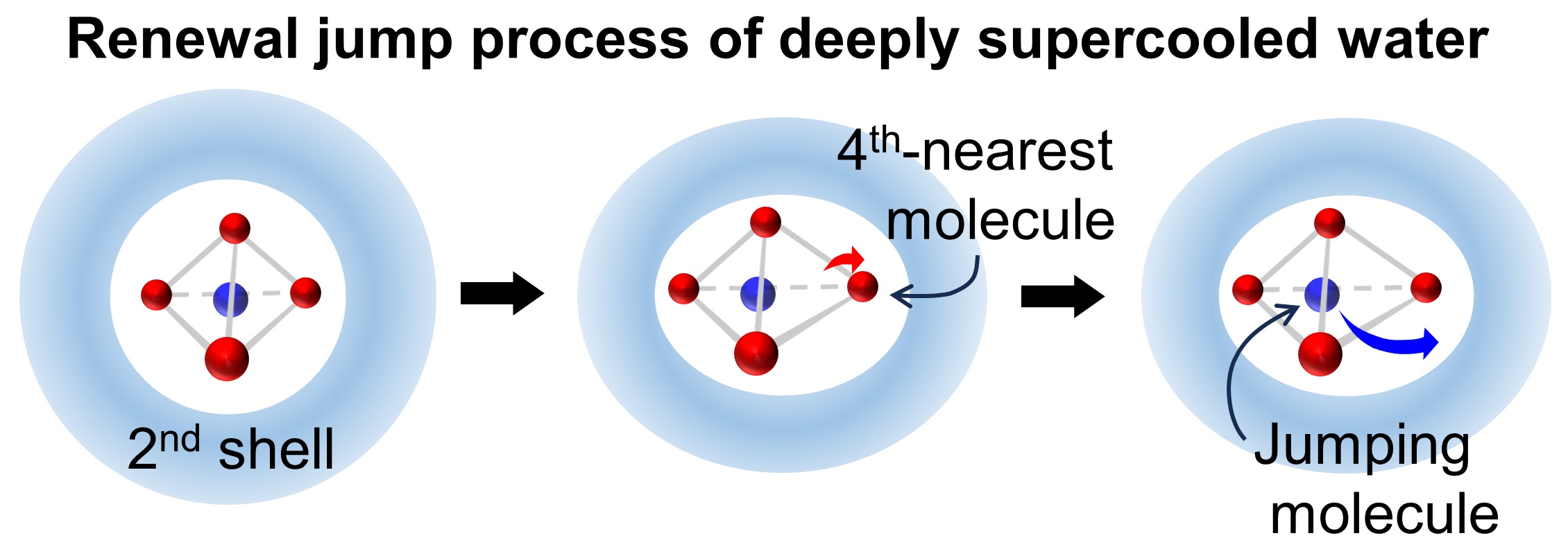
Unraveling the dynamic slowdown in supercooled water: The role of dynamic disorder in jump motions
When a liquid is rapidly cooled below its melting point without inducing crystallization, its dynamics slow down significantly without noticeable structural changes. Elucidating the origin of this slowdown has been a long-standing challenge. Here, we report a theoretical investigation into the mechanism of the dynamic slowdown in supercooled water, a ubiquitous yet extraordinary substance characterized by various anomalous properties arising from local density fluctuations. Using molecular dynamics simulations, we found that the jump dynamics, which are elementary structural change processes, deviate from Poisson statistics with decreasing temperature. This deviation is attributed to slow variables competing with the jump motions, i.e., dynamic disorder. The present analysis of the dynamic disorder showed that the primary slow variable is the displacement of the fourth nearest oxygen atom of a jumping molecule, which occurs in an environment created by the fluctuations of molecules outside the first hydration shell. As the temperature decreases, the jump dynamics become slow and intermittent. These intermittent dynamics are attributed to the prolonged trapping of jumping molecules within extended and stable low-density domains. As the temperature continues to decrease, the number of slow variables increases due to the increased cooperative motions. Consequently, the jump dynamics proceed in a higher-dimensional space consisting of multiple slow variables, becoming slower and more intermittent. It is then conceivable that with further decreasing temperature, the slowing and intermittency of the jump dynamics intensify, eventually culminating in a glass transition.
|
Thermodynamic picture of vitrification of water through complex specific heat and entropy: A Journey through ‘No Man’s Land’
We investigate thermodynamic properties of supercooled water across the “no man’s land” onto the formation of amorphous ice. The calculations are aided by very long computer simulations, often more than 50 μs long, with the TIP4P/2005 model potential. Density fluctuations that arise from the proximity to a putative liquid-liquid (LL) transition at 228 K, cast a long shadow on the properties of water, both above and below the LL transition. We carry out the calculations of the quantum mechanical static and frequency-dependent specific heats by combining seminal works by Lebowitz, Percus, and Verlet and Grest and Nagel with the harmonic approximation for the density of states. The obtained values are in quantitative agreement with all available experimental and numerical results of specific heats for both supercooled water and ice. We calculate the entropy at all the state points by integrating the specific heat. We find that the quantum corrected-contributions of intermolecular vibrational entropy dominate the excess entropy of amorphous phases over the crystal over a wide range of temperature. Interestingly, the vibrational entropy lowers the Kauzmann temperature, TK, to 130 K, just below the experimental glass-to-liquid water transition temperature, Tg, of 136 K and the calculated Tg of 135 K in our previous study. A straightforward extrapolation of high temperature entropy from 250 K to below however would give a much higher value of TK~190 K. The calculation of Lindemann ratios places the melting of amorphous ice ~135 K. The amorphous state exhibits an extremely short correlation length for the distance dependence of orientational correlation.

|
Crucial role of fragmented and isolated defects in persistent relaxation of deeply supercooled water
Properties of water have been well elucidated for temperatures above ~230 K and yet mysteries
remain in the deeply supercooled region. By performing extensive molecular dynamics simulations
on this supercooled region, we find that structural and dynamical instabilities are hidden in
the experimentally inaccessible region between 235 K and 150 K. We find a hitherto undiscovered
fragmentation from 220 K to 190 K, which is the break-up of large clusters consisting of
molecules with locally distorted tetrahedral structure into small pieces with one or two isolated
defects. The fragmentation leads to considerable changes in the relaxation dynamics of water.
We reveal a crucial role of specific three-coordinated defects in slow but persistent structural
relaxation. The presence of relaxation due to these specific defects makes water glass transition
temperature Tg (= 136 K) extremely low and explains why the Tg of water is ~1/2 of the melting
temperature Tm, much lower than the commonly obeyed 2/3 rule of Tg/Tm.

|
Frequency dependence of specific heat in supercooled liquid water and emergence of correlated
dynamics
Molecular origin of the well-known specific heat anomaly in supercooled liquid water is investigated here
by using extensive computer simulations and theoretical analyses. A rather sharp increase in the values
of isobaric specific heat with lowering temperature and the weak temperature dependence of isochoric
specific heat in the same range are reproduced in simulations. We calculated the spatio-temporal
correlation among temperature fluctuations and examined the frequency dependent specific heat.
The latter shows a rapid growth in the low frequency regime as temperature is cooled below 270 K.
In order to understand the microscopic basis of this increase, we have performed a shell-wise
decomposition of contributions of distant molecules to the temperature fluctuations in a central
molecule. This decomposition reveals the emergence, at low temperatures, of temporally slow, spatially
long ranged large temperature fluctuations. The temperature fluctuation time correlation function (TFCF)
can be fitted to a William-Watts stretched exponential form with the stretching parameter close to 0.6
at low temperatures, indicating highly non-exponential relaxation. Temperature dependence of the
relaxation time of the correlation function can be fitted to Vogel-Fulcher-Tamermann (VFT) expression
which provides a quantitative measure of the fragility of the liquid. Interestingly, we find that
the rapid growth in the relaxation time of TFCF with lowering temperature undergoes a sharp crossover
from a markedly fragile state to a weakly fragile state around 220 K.

|




